Need More Information?
To learn more about this 2-level FDA Approval or the prodisc C Vivo & prodisc C SK devices, please fill in the form.
NOTE: If you are a patient, learn more at the rediscover Patient Website.

 Learn More
Learn More
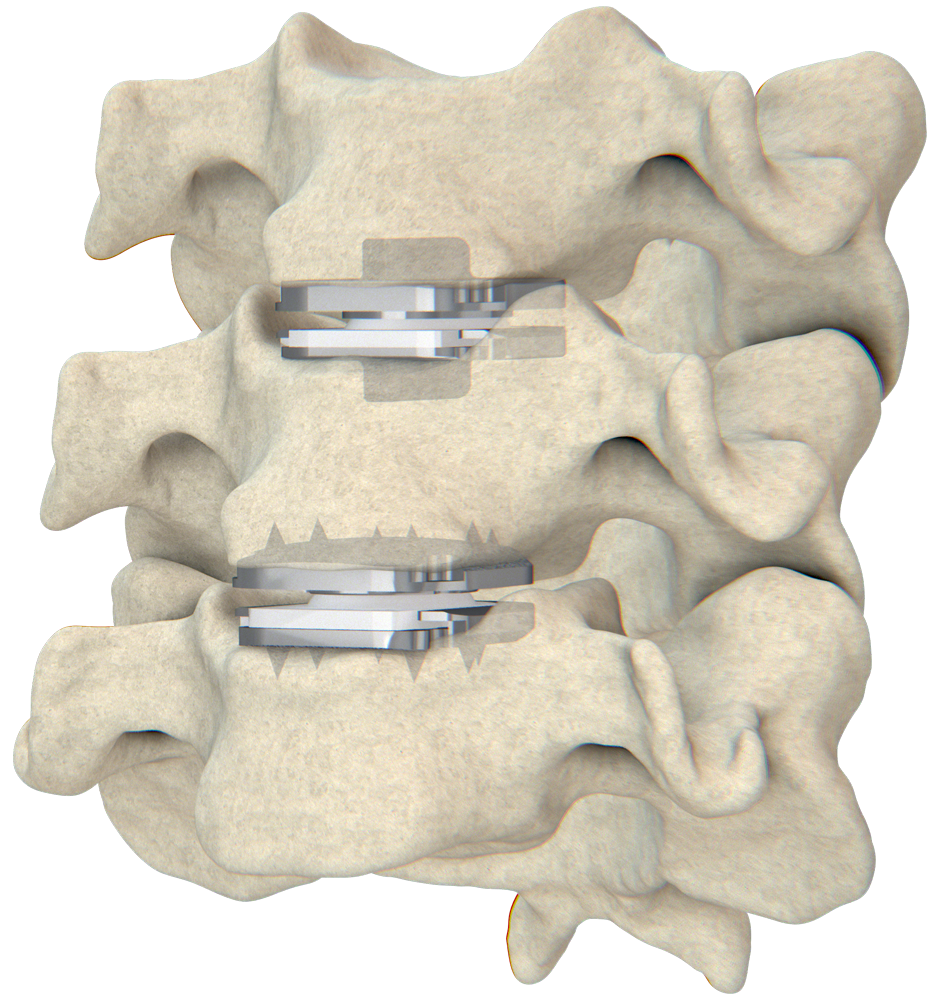
The prodisc C Vivo & prodisc C SK are now indicated for reconstruction of a vertebral disc from C3-C7 following discectomy at one level or two contiguous levels.
The prodisc C Vivo & prodisc C SK Match-the-Disc™ System is the first and only cervical TDR system with two different devices approved for both one- and two-level use.
Centinel Spine’s prodisc technology is now the only TDR solution in the U.S. approved for one- and two-level use in both the cervical and lumbar spine.


| prodisc C Vivo / SK | Mobi-C® | |
| Implanted | 291 | 140 |
| No secondary surgical intervention | 96.9% | 95.7% |
| No Neurological Deterioration*** (CEC) | 98.0% | 96.8% |
| No Major Adverse Event (CEC) | 99.7% | 100% |
| NDI 15-point Responder*** | 93.9% | 90.1% |
| Composite Clinical Success Results** | 87.1%* | 83.7% |
| FDA Approved Two-Level Cervical Disc | 24 Month PMA Study Composite Clinical Success Rate |
| prodisc C Vivo & prodisc C SK | 87.1% i |
| Simplify | 86.7% ii |
| Prestige LP | 81.4% iii |
| Mobi-C | 69.7% iv |
Watch this short primer on Centinel Spine and its unique and extraordinary place as a catalyst of change in the spine industry—with pioneering technologies and a clinical history that have led to successes ranging from PGA champions to a growing list of surgeon-patients.
 SEE MORE VIDEOS
SEE MORE VIDEOS