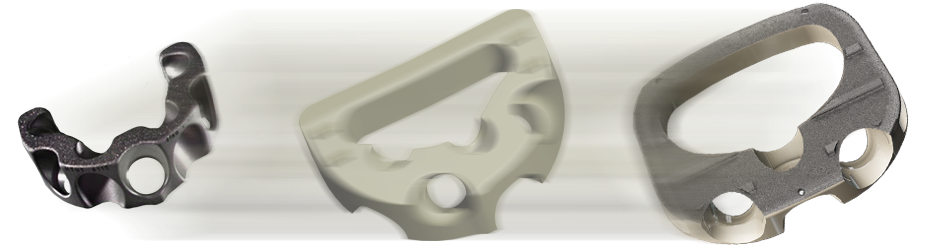
 Learn More About Integrated Interbody
Learn More About Integrated Interbody
 Toward the end of the 1970’s, Mr. John Dove, FRCS, studied under the guidance of Mr. O’Brien at Robert Jones & Agnes Hunt Hospital, Oswestry, England.
Toward the end of the 1970’s, Mr. John Dove, FRCS, studied under the guidance of Mr. O’Brien at Robert Jones & Agnes Hunt Hospital, Oswestry, England.


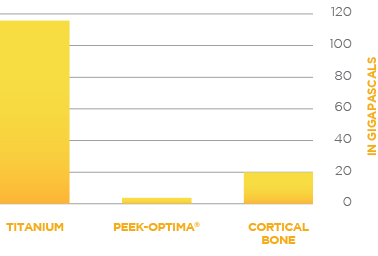



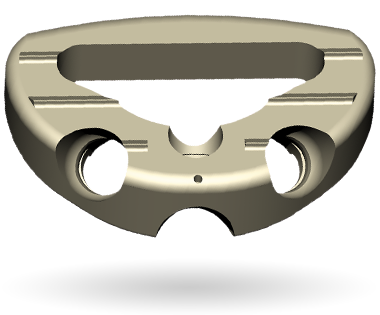


Watch this short primer on Centinel Spine and its unique and extraordinary place as a catalyst of change in the spine industry—with pioneering technologies and a clinical history that have led to successes ranging from PGA champions to a growing list of surgeon-patients.
 SEE MORE VIDEOS
SEE MORE VIDEOS